Intravenous immunoglobulin (IVIg)
Intravenous immunoglobulin (IVIg) is a solution of human plasma proteins and in particular IgG antibodies with a broad spectrum of antibody activity. IVIg is prepared from large pools of human plasma collected from several thousand blood donors. It’s used for patients who need replacement of antibodies and with autoimmune disorders.
IVIg can be obtained under National Blood Supply (NBS) or Jurisdictional Direct Order arrangements. Refer to the National Blood Authority website about the supply of IVIg in Australia.
Always read and refer to the product information sheet of every product prior to administration.
Criteria for the clinical use of intravenous immunoglobulin
Intravenous immunoglobulin (IVIg) is issued in accordance with the Criteria for the clinical use of immunoglobulin in Australia.
The criteria describe the conditions and circumstances for which IVIg is funded under the National Blood Supply (NBS) arrangements.
Access to intravenous immunoglobulin
In all states/territories, government-funded IVIg is accessed online by registered users of BloodSTAR.
BloodSTAR is the National Blood Authority’s online system that facilitates authorisation, dispensing and reviews of immunoglobulin products such as IVIg.
To register for BloodSTAR you will need to create an account with the National Blood Authority’s BLOODportal.
Further assistance with accessing immunoglobulins for patients is available on the National Blood Authority's website.
- Privigen® AU
Privigen® AU (Human Normal Immunoglobulin 10% [100 mg/mL] available in vial sizes 5g/50mL, 10g/100mL and 20g/200mL is an Australian normal immunoglobulin product manufactured by CSL Behring and accessed under the National Blood Supply (NBS) arrangements.
Refer to the Criteria for the clinical use of immunoglobulin in Australia for specific indications and product information sheet for further information.
Information on supply and pricing is outlined on the National Blood Authority website.
Privigen® AU product information
This link takes you to CSL Behring's company website where you can access information about Privigen® AU. You can download the product information (PI) sheet for this product from this webpage.
The PI details the pharmacology, indications, contraindications, precautions, adverse effects, use in pregnancy and lactation, dosage, and administration of Privigen® AU.
Lifeblood has sent a letter to all Approved Health Providers regarding the introduction of Privigen® AU and a copy can be found here.
More information about the transition to Privigen® AU can be found on CSL Behring’s website Resource Hub for Healthcare Professionals and on the National Blood Authority’s website.
- Privigen®
Privigen® is an imported IVIg product.
Privigen® is supplied by CSL Behring and available as a 10% solution in vial sizes: 5g/50 mL, 10g/100 mL, 20g/200 mL and 40g/400 mL.
Refer to the Criteria for the clinical use of immunoglobulin in Australia for specific indications and product information sheet for further information.
Information on supply and pricing is outlined on the National Blood Authority website.
This link takes you to CSL Behring's web information about Privigen® where you can download the product information (PI) sheet for this product.
The PI details the pharmacology, indications, contraindications, precautions, adverse effects, use in pregnancy and lactation, dosage and administration of Privigen®.
- Flebogamma®
Flebogamma® is an imported IVIg product. Flebogamma® is supplied by Grifols and available in both 5% and 10% formulations.
Refer to the Criteria for the clinical use of clinical immunoglobulin for specific indications and product information sheet for further information.
Information on supply and pricing is outlined on the National Blood Authority website.
Flebogamma® 5% DIF
Flebogamma® 5% DIF is available in vial sizes: 5g/100 mL, 10g/200 mL and 20g/400 mL.
Flebogamma® 5% product information
This link takes you to Grifol's web information about Flebogamma® 5% DIF where you can download the product information (PI) sheet for this product.
The PI details the pharmacology, indications, contraindications, precautions, adverse effects, use in pregnancy and lactation, dosage and administration of Flebogamma® 5% DIF.
Phasing out of Flebogamma 5% DIF vial size 2.5g/50 mL
From September 2025, Grifols is phasing out Flebogamma 5% DIF 2.5g/50 mL from Australia’s national blood arrangements. The remaining inventory of this vial size is expected to be exhausted from mid-September 2025.
For more information, please refer to the customer communications from Lifeblood and the National Blood Authority.
Flebogamma® 10% DIF
Flebogamma® 10% DIF is available in vial sizes: 5g/50 mL, 10g/100 mL and 20g/200 mL.
Flebogamma® 10% product information
This link takes you to Grifol's web information about Flebogamma® 10% DIF where you can download the product information (PI) sheet for this product.
The PI details the pharmacology, indications, contraindications, precautions, adverse effects, use in pregnancy and lactation, dosage and administration of Flebogamma® 10% DIF.
- Gamunex®
Gamunex® is an imported IVIg product. Gamunex® is supplied by Grifols and available as a 10% solution in vial sizes: 5g/50 mL, 10g/100 mL, 20g/200 mL and 40g/400mL.
Refer to the Criteria for the clinical use of immunoglobulin in Australia for specific indications and product information sheet for further information.
Information on supply and pricing is outlined on the National Blood Authority website.
This link takes you to Grifol's web information about Gamunex® where you can download the product information (PI) sheet for this product.
The PI details the pharmacology, indications, contraindications, precautions, adverse effects, use in pregnancy and lactation, dosage and administration of Gamunex®.
- Octagam® 10%
Octagam® 10% is an imported IVIg product. Octagam® 10% is supplied by Octapharma and available as a 10% solution in vial sizes: 5 g/50 mL, 10 g/100 mL and 20 g/200 mL.
Refer to the Criteria for the clinical use of immunoglobulin in Australia for specific indications and product information sheet for further information.
Information on supply and pricing is outlined on the National Blood Authority website.
Octagam® 10% product information
This link takes you to Octapharma's PI for Octagam® 10%.
The PI details the pharmacology, indications, contraindications, precautions, adverse effects, use in pregnancy and lactation, dosage and administration of Octagam® 10%.
- Kiovig®
Kiovig® (Human Normal Immunoglobulin 10% [100 mg/mL] is an imported IVIg product accessed under the National Blood Supply (NBS) arrangements.
Kiovig® is supplied by Takeda Pharmaceuticals Australia and available in vial sizes: 5g/50mL, 10g/100mL, 20g/200mL and 30g/300mL.
Refer to the Criteria for the clinical use of immunoglobulin in Australia for specific indications and product information sheet for further information.
Information on supply and pricing is outlined on the National Blood Authority website.
Kiovig® product information
This link takes you to Takeda Pharmaceuticals Australia company website where you can access information about Kiovig®. You can download the product information (PI) sheet for this product from this webpage.
The PI details the pharmacology, indications, contraindications, precautions, adverse effects, use in pregnancy and lactation, dosage, and administration of Kiovig®.
Lifeblood has sent a letter to all Approved Health Providers regarding the reintroduction of Kiovig® and a copy can be found here.
Further information
The Australian Immunisation Handbook recommends that immunoglobulin containing blood products, apart from RhD Immunoglobulin (anti-D), should not be administered for 3 weeks following vaccination with live attenuated vaccines. MMR containing vaccines should not be given for 3-11 months following the receipt of immunoglobulin containing blood products. Further information regarding all vaccines should be obtained from the Australian Immunisation Handbook.
- Supply of plasma derived and recombinant blood products fact sheet
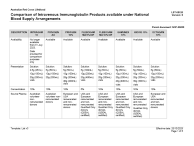
- Download the comparison of Intravenous Immunoglobulin Products available under National Blood Supply arrangements
Updated October 2025