Component labelling
Blood and blood products are regulated by the Therapeutic Goods Administration (TGA) under the Therapeutic Goods Act, and the component label is part of those regulatory requirements. The label uniquely links the donation to the donor to ensure traceability and contains other mandatory information about the product.
ISBT 128 labelling
In 2018, Lifeblood moved to the ISBT 128 (Information Standard for Blood and Transplant) labelling standard for blood component labels.
The ISBT 128 labelling standard is internationally recognised and improves blood component traceability for the safety of patients and donors.
Blood component labels contain clinically important information specific to each individual unit. These labels can also have modifier texts, such as red cell phenotype or CMV antibody status.
Remember to check labels prior to transfusion to ensure that the unit meets the transfusion requirements of the patient.
Right patient, right product, right time.
ISBT 128 transition label
With the move to the ISBT 128 standard, blood component labels will change with the introduction of a new ISBT 128 ‘Component Transition Label’ (shown below). This will include both ISBT 128 barcodes as well as the current Codabar linear barcodes, allowing health providers who do not yet have ISBT 128 capability to continue managing their inventory without interruption.
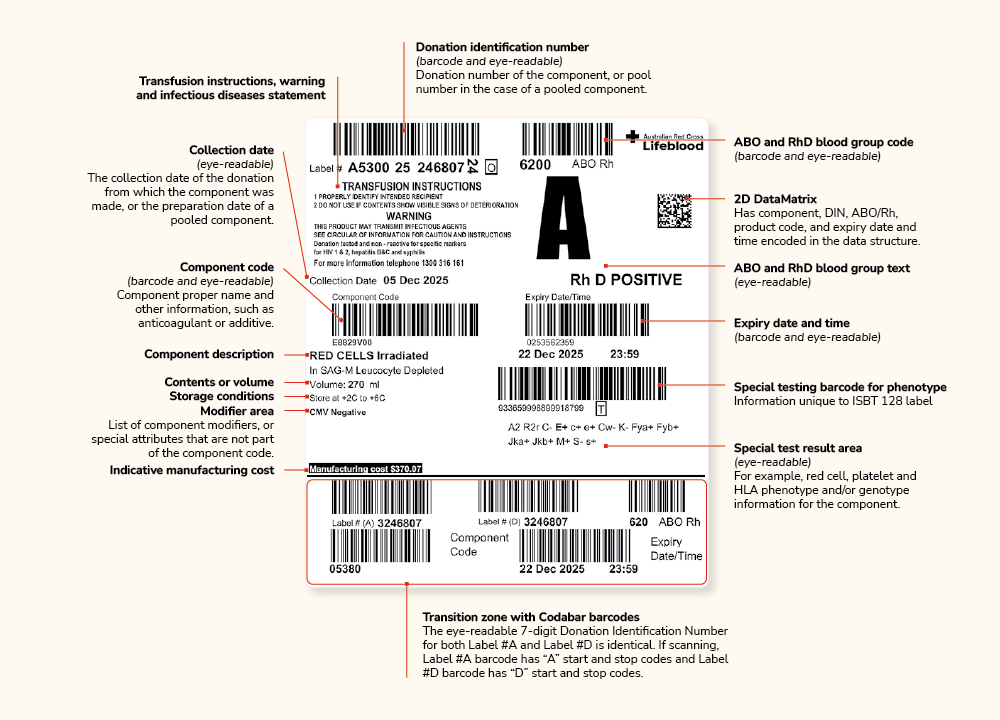
Codabar label
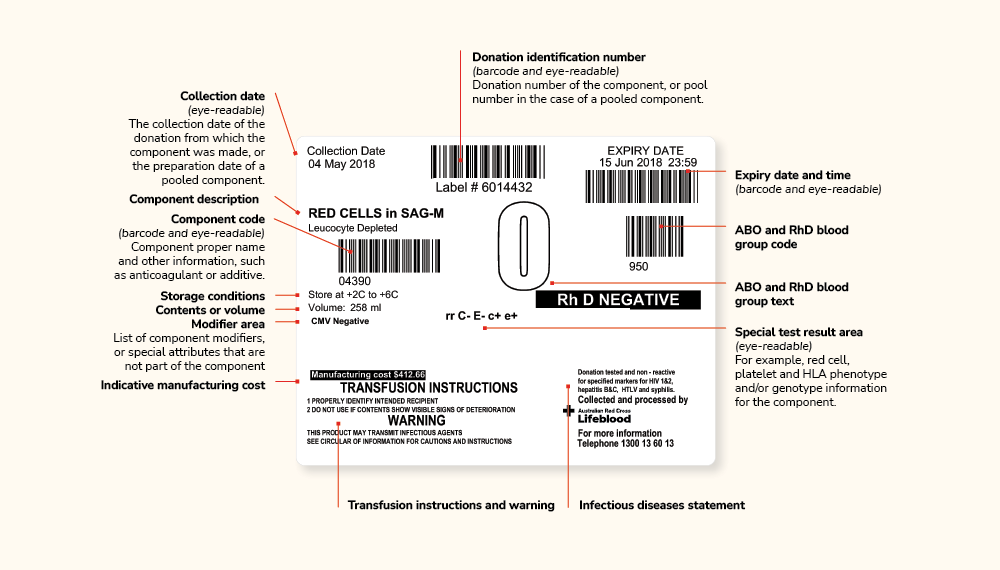
The label format (shown in the image below) was the Codabar linear format used prior to 2018. Frozen red cells produced prior to this time will have Codabar labelling. You can find specific details about Codabar labels in the Label and Component Information document.
Please send any queries to your local transfusion scientist.
Check Character Calculation
Donations Identification Number Calculator Tool
The Donations Identification Number (DIN) Calculator Tool can be used to convert Transition CODABAR donation numbers from blood components into ISBT128 donation numbers.
CAUTION: At Lifeblood, donations collected in:
- December of a current year, e.g. 2023, may be assigned an ISBT128 DIN for either the current year (A530023 ...) OR the upcoming year 2024 (A530024 ...).
- January of a new year, e.g. 2024, may be assigned an ISBT128 DIN for either the new year (A530024 ...) OR the previous year (A530023…).
For components collected in December or January of any year, contact Lifeblood for assistance in identifying the related ISBT128 number when required.
The tool can also be used to find the ISBT128 manual check character.
Updated August 2025




