Milk program
Lifeblood's milk program
Lifeblood’s pasteurised donor human milk (PDHM) program is part of our commitment to deliver new and enhanced health products and services to achieve improved health outcomes.
PDHM is made from breast milk that’s been donated to Lifeblood by voluntary, non-remunerated mothers living in Brisbane, Sydney, Melbourne and Adelaide, who are expressing more milk than their own baby needs.
PDHM is the preferred alternative source of nutrition for very preterm infants where a mother’s own milk is not available.
We currently supply PDHM to >40 Neuonatal Intensive Care Units and Special Care Nurseries across the country.
- Why is it needed?
In Australia, 26,000 babies are born preterm each year, with around 4,000 born very preterm or very low birth weight. It’s estimated 70-80% of infants in neonatal intensive care don’t have access to sufficient maternal milk and require supplementary nutrition.
- What are the benefits?
When sufficient maternal milk is not available, using PDHM instead of infant formula as supplementary nutrition reduces the risk of necrotising enterocolitis by around half for infants born very preterm (<32 weeks’ gestation) or very low birthweight (<1,500 g).
- Clinical use
PDHM is primarily used as supplementary nutrition for babies born <32 weeks’ gestation, weighing <1,500 g, or at clinician discretion, when there is insufficient maternal milk supply.
PDHM is not equivalent to maternal breast milk and does not replace the need for maternal lactation support.
Lifeblood also participates research into the benefits and risks of PDHM in other cohorts, providing PDHM for multi-centre randomised controlled clinical trials of PDHM for moderate-late preterm infants, and for term infants of women with diabetes in pregnancy.
- Processing and testing
Lifeblood receives donor milk from voluntary non-remunerated donors with a supply that exceeds the needs of their own infant. About a third of our donors are parents of preterm infants. Prospective donors express their breast milk and store it in their freezer.
If the donor passes the high-level screening and donor questionnaire, Lifeblood donor coordinators visit the prospective donors at their home, hospital or workplace. Using a formal donor questionnaire with signed declaration, they ensure that donors meet the strict selection criteria to be able to donate breast milk. Blood tests are then collected at the donor’s home and the team brings the frozen donor milk back to Lifeblood processing centres in validated cold shipping containers.
Donors have infectious diseases testing done once per lactational period. They’re tested for HIV, Hepatitis B, Hepatitis C, HTLV and syphilis using serology, with additional nucleic acid testing for HIV, Hepatitis B and Hepatitis.
Once donor breast milk is received in the processing centre, the frozen milk is thawed to form a batch. It’s aliquoted into BPA-free bottles, which are cap sealed prior to Holder pasteurisation. After pasteurisation, the milk is stored frozen ready for distribution.
Holder pasteurisation (62.5 ºC for 30 minutes) inactivates:
- HIV, CMV and many other viruses, and
- most bacterial pathogens.
Spore forming bacteria and some bacterial toxins may survive pasteurisation, so each batch is tested for bacteria before and after pasteurisation, with strict microbial acceptance criteria.
This testing is done in a NATA accredited food safety laboratory prior to release of PDHM to hospitals.
Pre-pasteurisation criteria
- ≤105 cfu/mL total microorganism count
- ≤104 cfu/mL Enterobacteriaceae
- ≤104 cfu/mL Staphylococcus aureus
Post-pasteurisation criteria
≤1 cfu/mL total microorganism count
- Storage and use
PDHM has a 3-month expiry date from date of pasteurisation.
PDHM is stored frozen and must be discarded 24 hours after thawing.
- Nutritional information
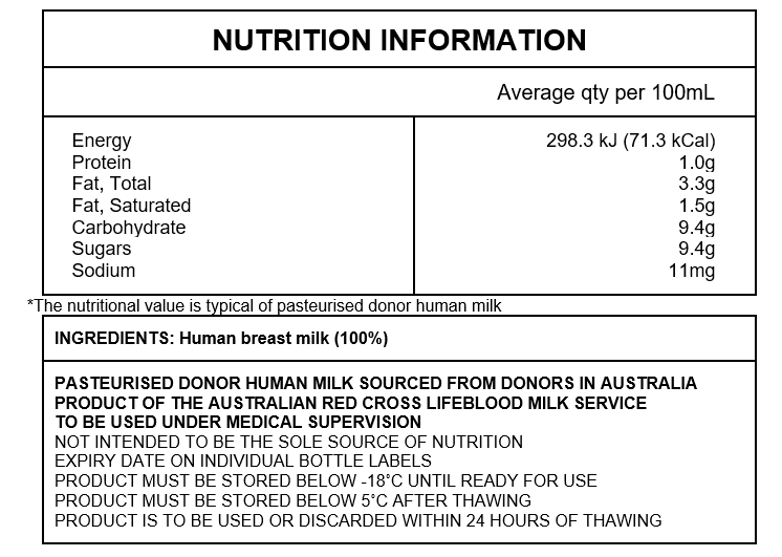
- How do we ensure donor and product safety?
Lifeblood operates their milk program under Food Standards Australia New Zealand (FSANZ) regulation, with a comprehensive food safety plan based on a hazards analysis critical control point quality framework.
This includes the screening and testing described above, as well as environmental screening, adverse event surveillance, surveillance of emerging and re-emerging infections, and milk safety research.
Contact us
For enquiries about Lifeblood’s milk program, call 1300 459 040.
High-level screening eligibility
A donor will not be eligible if:
- they have smoked or used nicotine replacement therapy in the last 6 months
- they have used recreational drugs in the past 12 months
- they have ever injected drugs
- they drink alcohol regularly
- they have HlV, hepatitis B or C, HTLV or syphilis
- they are under 18 years of age, and
- their baby is older than 12 months.






